 产业资讯
产业资讯
 复宏汉霖
复宏汉霖  2024-03-22
2024-03-22
 977
977
2024年3月21日,复宏汉霖(2696.HK)发布2023年度业绩,于业绩期内实现营业收入约人民币53.949亿元,较去年同期增长约67.8%,净利润达5.460亿元,这是继2023上半年首次实现半年度盈利之后,公司首次实现全年盈利。此次盈利主要源于公司核心产品陆续实现商业化销售后持续销量扩大,2023年产品销售收入合计约人民币45.535亿元,同比增长70.2%。
截至目前,复宏汉霖已有5款产品在中国上市,2款产品全球上市,19项适应症获批,触达超过40个国家和地区,广泛覆盖亚洲、欧洲、拉丁美洲和大洋洲,并惠及逾56万名患者。此外,公司现有50余个上市注册申请获中国、欧盟、美国、加拿大、新加坡、泰国等多个国家和地区药品监管部门受理,加速产品全球化进程。同时,公司秉持差异化的创新策略,持续丰富并优化产品管线,2023年度研发投入约人民币14.336亿元。

复宏汉霖董事长、执行董事张文杰
2023年,复宏汉霖首次实现全年盈利,取得了令人瞩目的业绩。依靠不懈的创新精神和卓越的执行能力,我们不断推动一体化生物制药平台性能提升,全面筑牢高质量发展根基,赋能公司跑出发展‘加速度’。同时,我们深化经营策略和管理流程,多维提升运营效能,为公司持续发展提供有力支撑。复宏汉霖正稳步向成为高质量发展的国际化创新生物制药企业的宏伟目标迈进。

复宏汉霖执行董事、首席执行官兼首席财务官朱俊
在‘生物类似药和创新药’双轮驱动战略下,我们聚焦临床未被满足的需求,持续创新攻关、加速国内外市场拓展、坚持精益化运营,推动产品协同高质发展,公司综合竞争实力再进阶。未来,我们将继续秉持‘以患者为中心’的理念,深化创新布局,加速高品质生物药惠及全球更多患者。
商业效能高增长,出海再迎新突破
2023年,公司全面推进产品商业化进程,持续构建创新商业模式、优化市场布局并拓宽海外市场,公司5款产品实现销售收入合计约人民币45.535亿元。其中,公司自营产品汉曲优®(曲妥珠单抗,欧洲商品名:Zercepac®)、汉斯状®(斯鲁利单抗)和汉贝泰®(贝伐珠单抗)分别达成全年销售收入人民币27.370亿元、11.198亿元、1.194亿元。此外,基于与合作伙伴的约定,公司就汉利康®(利妥昔单抗)、汉达远®(阿达木单抗)分别获得销售收入约人民币5.405亿元和0.586亿元。

公司抗肿瘤核心产品汉曲优®延续强劲增长势头,其中,国内市场份额持续攀升,全年达成销售收入约人民币26.444亿元,同比增长56.1%。凭借150mg和60mg双规格、不含防腐剂等差异化优势,汉曲优®产品人群覆盖不断扩大,迄今已惠及超过18万名中国患者。2023年,汉曲优®海外市场销售收入进一步提高,约人民币0.926亿元,同比增长162.3%。作为国产生物药“出海”代表,汉曲优®已于40余个国家和地区获批上市,包括英国、德国、西班牙、法国、意大利、瑞典、澳大利亚、新加坡、阿根廷、巴西等,是获批上市国家和地区最多的国产生物类似药,同时,该产品还被纳入中国、英国、法国和德国等国家医保,进一步提升可及性。2023年,汉曲优®海外商业化版图成功新添泰国、菲律宾和巴西市场,其于美国、加拿大的上市申请亦已获得受理,有望于2024年获批上市,进一步惠及全球更多患者。
公司自主研发创新型单抗H药 汉斯状®是全球首个一线治疗小细胞肺癌的抗PD-1单抗,并已在中国和印尼获批上市。2023年,该产品达成销售收入约人民币11.198亿元,同比增长230.2%。目前,H药已获批4项适应症,2023年新增获批广泛期小细胞肺癌(ES-SCLC)和食管鳞状细胞癌(ESCC),迄今已惠及6万余名中国患者,该产品第5项适应症一线治疗非鳞状非小细胞肺癌(nsNSCLC)的上市注册申请也已获国家药品监督管理局(NMPA)受理。另一方面,H药的全球化步伐也在不断加速,截至目前H药对外授权已覆盖美国、欧洲、东南亚、中东和北非等70多个国家和地区。2023年12月,H药于印度尼西亚获批上市,成为首个在东南亚国家成功获批上市的国产抗PD-1单抗。同时,公司于泰国、新加坡、马来西亚等国家递交了H药上市许可申请,进一步推动H药在东南亚地区的上市进程。在欧美生物药市场,H药一线治疗ES-SCLC的欧盟上市许可申请(MAA)于2023年3月获得欧洲药品管理局(EMA)受理,同时,复宏汉霖稳步推进H药对比一线标准治疗阿替利珠单抗用于治疗ES-SCLC的头对头美国桥接试验,以进一步支持其在美国的上市申报。此外,公司全面推进基于H药的肿瘤免疫联合疗法,并在全球同步开展10余项免疫联合疗法临床试验。

2023年,公司商务合作取得多项里程碑式进展,激发产品“出海”新活力,共获得授权许可及其他收入约人民币8.414亿元,同比增长56.0%。业绩期内,公司与Boston Oncology就汉利康®达成在中东北非区域16个国家的商业化合作;公司再次携手KGbio就H药在中东北非地区12个国家的独家开发和商业化权益达成合作;复宏汉霖与Intas合作,授权其H药在欧洲和印度共50多个国家的独家开发和商业化权益。截至目前,公司就汉利康®、汉曲优®、H药等8个产品,携手Accord、Abbott、Eurofarma、Elea和KGbio等国际一流的生物制药企业拓展全球市场,全面覆盖欧美主流生物药市场和众多新兴市场。
守正创新绘新图 提质增效再升级
作为一家国际化的创新生物制药公司,复宏汉霖始终以临床需求为导向,协同全球创新中心和产品开发团队,持续加码创新,缔造高质量、可负担且具有差异化优势的产品管线,切实满足患者和市场需求。截至目前,公司产品管线已涵盖超过50个分子,覆盖抗体、抗体偶联药物、融合蛋白、小分子药物等药物形式,并同步就16个产品在全球范围内开展30多项临床试验。
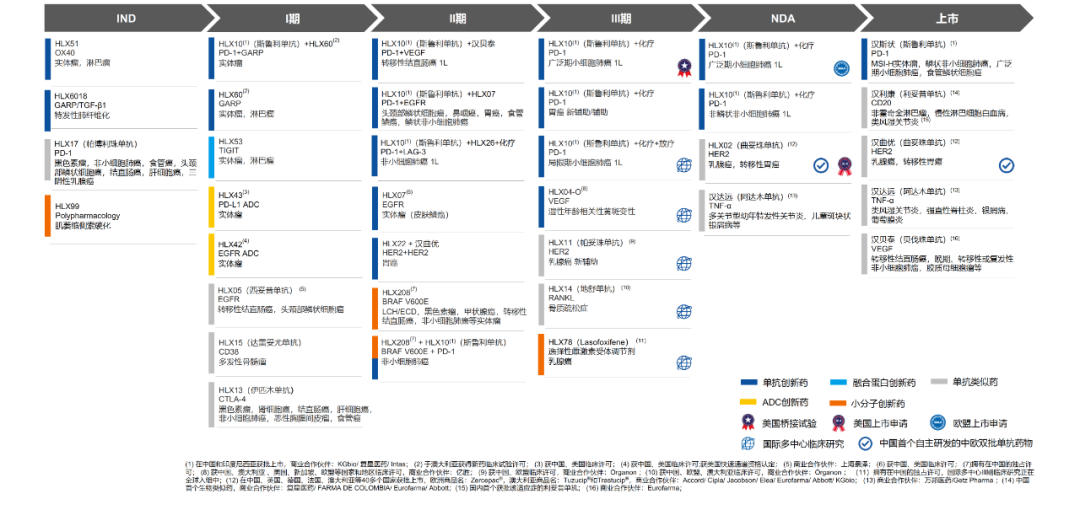
2023年,公司在全球范围内加速推进包括H药、HLX11(帕妥珠单抗生物类似药)、HLX14(地舒单抗生物类似药)和HLX04-O(抗VEGF单抗)等多个已上市或临床后期阶段产品的国际多中心III期临床研究,并计划于2024年在全球范围内递交相关上市申请。另一方面,H药、HLX208(BRAFV600E小分子抑制剂)、HLX07(抗EGFR单抗)、HLX22(抗HER2单抗)、HLX26(抗LAG-3单抗)、HLX42(EGFR ADC)和HLX43(PD-L1 ADC)等多款创新产品研究结果荣登Nature Medicine、Cancer Cell等权威医学期刊以及2023 ASCO、2023 ESMO、2023 ESMO Asia等国际学术舞台,获得广泛认可和高度评价。
此外,复宏汉霖积极探索新靶点、新机制,以抗肿瘤药物为基石,基于人群疾病谱不断拓展治疗领域和新分子类型,加速针对复杂疾病的创新药物研发。2023年,公司全力推进HLX42、HLX43、HLX6018(创新抗GARP/TGF-β1单抗)和HLX99(多重药理学小分子)等多款潜在first/best-in-class产品进入临床/临床注册申报阶段,并成功获得多款产品的突破性疗法认定和快速通道资格认定。同时,公司亦加速引进与现有管线具有乘数叠加效应的创新产品,以持续激发增长潜力。近期,公司获得了一款处于全球III期临床阶段的新型乳腺癌内分泌疗法lasofoxifene的中国权益,加速为更多患者提供更加有效、精准化的治疗方案。

2023年,复宏汉霖赓续综合一体化生产平台建设,持续打造全球供应体系,助力公司全球商业化布局,为普惠患者提供有力保障。公司现有上海徐汇、松江(一)和松江(二)三大生产基地,目前商业化总产能达48,000升,已实现中国、欧洲、东南亚和拉美等市场的常态化供应,并于2023年完成松江基地(二)两幢主要生产楼竣工验收和首批工程批生产。公司对标国际最高标准构建质量管理体系,已通过近百项由各国药监机构以及国际合作伙伴实施的实地核查或审计,其中徐汇基地和松江基地(一)均已获得中国和欧盟GMP认证。2023年,公司生产基地先后就汉曲优®、H药、汉利康®等重磅产品获得药品检查合作计划(PIC/S)成员印尼和巴西GMP认证,并就H药相关产线获欧盟GMP认证;同年,公司松江基地(一)还接受了美国FDA对汉曲优®的上市许可前检查。
复宏汉霖始终秉持“以患者为中心”的理念,未来将进一步夯实biopharma商业化能力、深化全球战略布局,推动高质量创新研发,并持续提升生产运营质量和效率,实现从高速增长到高质量发展的跨越,让更多、更高质量的创新成果惠及全球更多患者。
关于复宏汉霖
复宏汉霖(2696.HK)是一家国际化的创新生物制药公司,致力于为全球患者提供可负担的高品质生物药,产品覆盖肿瘤、自身免疫疾病、眼科疾病等领域,已在中国上市5款产品,在国际上市2款产品,19项适应症获批,7个上市申请分别获中国药监局、美国FDA和欧盟EMA受理。自2010年成立以来,复宏汉霖已建成一体化生物制药平台,高效及创新的自主核心能力贯穿研发、生产及商业运营全产业链。公司已建立完善高效的全球创新中心,按照国际药品生产质量管理规范(GMP)标准进行生产和质量管控,不断夯实一体化综合生产平台,其中,上海徐汇基地和松江基地(一)均已获得中国和欧盟GMP认证。
复宏汉霖前瞻性布局了一个多元化、高质量的产品管线,涵盖50多个分子,并全面推进基于自有抗PD-1单抗H药汉斯状®的肿瘤免疫联合疗法。继国内首个生物类似药汉利康®(利妥昔单抗)、中国首个自主研发的中欧双批单抗药物汉曲优®(曲妥珠单抗,欧洲商品名:Zercepac®)、汉达远®(阿达木单抗)和汉贝泰®(贝伐珠单抗)相继获批上市,创新产品汉斯状®(斯鲁利单抗)已获批用于治疗微卫星高度不稳定(MSI-H)实体瘤、鳞状非小细胞肺癌、广泛期小细胞肺癌和食管鳞状细胞癌,并成为全球首个获批一线治疗小细胞肺癌的抗PD-1单抗。公司亦同步就16个产品在全球范围内开展30多项临床试验,对外授权全面覆盖欧美主流生物药市场和众多新兴市场。
Henlius 2023 Annual Results: Revenue surpassed RMB5.39 billion with a net profit RMB546 million, first full-year profitability achieved
Shanghai, China, March 21st, 2024 – Henlius (2696.HK) announced its 2023 annual results. During the reporting period, Henlius’ revenue reached about RMB5.3949 billion, representing an increase of 67.8% YoY, and recorded a net profit of RMB546.0 million. This is the first time for Henlius to achieve full-year profits following the company’s achievement of its first half-year profits in H1 2023. The full year of profitability is due to increasing commercial sales of the core products and expanding sales volume. Henlius' products achieved total sales of approximately RMB4.5535 billion in 2023, up by 70.2% YoY.
Up to date, Henlius has 5 products launched in China, 2 launched in overseas markets, 19 indications approved worldwide, benefiting over 560,000 patients and reaching more than 40 markets in Asia, Europe, Latin America and Oceania. Besides, over 50 marketing applications of its products have been accepted for review in countries and regions including China, the EU, the U.S., Canada, Singapore and Thailand. Meanwhile, the company stays focused on differentiated innovation to accelerate the development of products in its pipeline. In 2023, the company's R&D expenditure reached approximately RMB1.4336 billion.
Wenjie Zhang, Chairman and Executive Director of Henlius, said: "Henlius achieved remarkable performance and recorded full-year profits for the first time. Through unwavering innovation and outstanding execution, we have established a solid foundation for high-quality development and have been continuously improving the efficiency of our integrated biopharmaceutical platform, enabling the company to accelerate its development. Furthermore, we've boosted efficiency in operations and management across the business, driving steady and strong growth and inspiring us to build an innovative global biopharmaceutical company with high-quality development."
Jason Zhu, Executive Director, Chief Executive Officer and Chief Financial Officer, said: "Under the dual-drive strategy of ‘biosimilars and novel biologics’, Henlius continues to accelerate both domestic and overseas market expansion, stick to lean operations, promote high-quality and synergistic product development, increase profitability and further advance its comprehensive capabilities. In the future, we will adhere to patient-centricity, further optimize our innovation layout, expand the global distribution of high-quality biological drugs, and make greater contributions to improving human health and well-being."
Achieving strong growth in commercial performance and making new breakthroughs in overseas expansion
In 2023, Henlius has made achievements in product commercialization, business models construction, market layout optimization and overseas market expansion. Its five products achieved total sales revenue of approximately RMB4.5535 billion. HANQUYOU (trastuzumab, trade name in Europe: Zercepac®), HANSIZHUANG (serplulimab) , and HANBEITAI (bevacizumab) recorded sales of RMB2.7370 billion, RMB1119.8 million and RMB119.4 million, respectively. In addition, the company received sales revenues of RMB540.5 million and RMB58.6 million based on the collaboration with partners for HANLIKANG (rituximab) and HANDAYUAN (adalimumab), respectively.
Henlius' core oncology product HANQUYOU continues to unleash strong growth momentum. During the reporting period, HANQUYOU has recorded a domestic sales revenue of RMB2.6444 billion, up by 56.1% YoY. Due to its advantages of 150mg/60mg dual dosage and preservative-free formulation, HANQUYOU further expanded its market share in China, benefiting over 180,000 Chinese patients to date. Overseas revenue from product sales recorded RMB92.6 million approximately, up by 162.3% YoY. As one of the pioneers of domestic biopharmaceuticals going global, HANQUYOU has successfully been approved for marketing in over 40 countries and regions, including the United Kingdom, Germany, Spain, France, Italy, Switzerland, Australia, Singapore, Argentina, Brazil, etc., becoming the China-developed biosimilar with the most marketing approvals. Meanwhile, the product’s accessibility has been further improved. It is now reimbursed nationally in countries including China, the UK, France, and Germany. In 2023, the overseas commercialization of HANQUYOU managed to include the markets of Thailand, the Philippines and Brazil, and its marketing applications in the United States and Canada have also been accepted and expected to be approved for marketing in 2024.
Henlius’ innovative product HANSIZHUANG, the world’s first anti-PD-1 monoclonal antibody (mAb) approved for the first-line treatment of small cell lung cancer (SCLC), has been launched in China and Indonesia. In 2023, HANSIZHUANG has recorded a total sales revenue of RMB1119.8 million, representing an increase of 230.2% YoY. Up to now, it has been approved for 4 indications and in 2023, it was approved for the treatment of extensive-stage small cell lung cancer (ES-SCLC) and esophageal squamous cell carcinoma (ESCC), benefiting over 60,000 Chinese patients to date. Additionally, the 5th NDA of HANSIZHUANG for the first-line treatment of non-squamous non-small cell lung cancer (nsNSCLC) has been accepted by the National Medical Products Administration (NMPA). On the other hand, Henlius continues to expand HANSIZHUANG’s global footprint, which now covers more than 70 countries and regions including the U.S., Europe, Southeast Asia and MENA. In December 2023, HANSIZHUANG was approved for marketing in Indonesia, becoming the first China anti-PD-1 mAb successfully approved in Southeast Asia. Furthermore, the company submitted marketing applications for HANSIZHUANG in Thailand, Singapore, Malaysia to further promote the product in Southeast Asia. In March 2023, HANSIZHUANG’s Marketing Authorization Application (MAA) for ES-SCLC has been validated by the European Medicines Agency (EMA). Moreover, Henlius has initiated a head-to-head bridging trial in the United States to compare HANSIZHUANG to standard of care atezolizumab (anti-PD-L1 mAb) for the first-line treatment of ES-SCLC to further support the Biologic License Application (BLA) in the U.S. Henlius is also advancing HANSIZHUANG-based tumour immuno-combination therapies and has initiated over 10 clinical trials worldwide.
In 2023, the company achieved a number of milestones in business development, boosting new vitality in global expansion for its products. During the reporting period, Henlius earned approximately RMB841.4 million in overseas licensing and other revenues, an increase of 56.0% YoY. In 2023, the company reached commercialization collaboration with Boston Oncology for HANLIKANG in 16 Middle East and North Africa (MENA) countries. Moreover, the company expanded its collaboration with KGbio to commercialize HANSIZHUANG in 12 MENA countries. It also deepened collaboration with Intas to commercialize HANSIZHUANG in over 50 countries in Europe and India. Up to now, the company has collaborated with international partners such as Accord, Abbott, Eurofarma, Elea and KGbio to globalize its 8 products including HANLIKANG, HANQUYOU and HANSIZHUANG, covering mainstream biopharmaceutical markets in Europe, the United States, and numerous emerging markets, further expanding its global presence.
Charting innovation and improving global supply with enhanced quality and efficiency
As an innovative global biopharma company, Henlius has always been guided by clinical needs and collaborated with its global innovation centres and product development teams to continuously boost innovation and build up a high-quality, affordable and differentiated pipeline to effectively meet the needs of patients and the market. Currently, its product pipeline covers more than 50 molecules including mAb, antibody-drug conjugate (ADC), fusion protein, and small molecule drug, and has conducted over 30 clinical studies for 16 products worldwide.
In 2023, the company has accelerated many international multi-centre phase 3 clinical researches of HANSIZHUANG, HLX11 (pertuzumab biosimilar), HLX14 (denosumab biosimilar) and HLX04-O (anti-VEGF mAb), with plans to submit marketing applications worldwide in 2024. On the other hand, the latest clinical results for HANSIZHUANG, HLX208 (BRAFV600E small molecule inhibitor), HLX07 (anti-EGFR mAb), HLX22 (anti-HER2 mAb), HLX26 (anti-LAG-3 mAb), HLX42(EGFR ADC)and HLX43(PD-L1 ADC)have been presented to global academic community, including Nature Medicine, Cancer cell, 2023 ASCO, 2023 ESMO, 2023 ESMO Asia and gained wide recognition.
Moreover, the company is actively exploring novel targets and mechanisms, constantly expanding into more disease areas and molecular types, so as to accelerate the development of innovative medicines to address complex diseases. Henlius is promoting a number of potential first/best-in-class products, including HLX42 , HLX43 , HLX6018 (anti-GARP/TGF-β1 mAb) and HLX99 (polypharmacology) to enter into clinical/clinical registration filing stage. The company has successfully obtained breakthrough therapy designation (BTD) and fast track designation (FTD) for a number of products. The company is also accelerating the introduction of innovative products that have multiplier effects with its existing pipeline to drive growth, and has recently acquired the China rights of a novel endocrine therapy for breast cancer, lasofoxifene, which is currently in global phase 3 clinical development, bringing more effective and targeted solutions to more patients.
In 2023, Henlius continued building an integrated and comprehensive production platform, enhancing its global supply system to support the company's worldwide commercial layout and to provide inclusive healthcare for patients. The company currently has three manufacturing facilities: Shanghai Xuhui Facility, Songjiang First Plant and Songjiang Second Plant. The current commercial capacity is 48,000 litres, enabling stable supply to markets beyond China, including Europe, Southeast Asia and Latin America. In 2023, the Songjiang Second Plant completed the construction and validation of two major buildings and the first engineering run. Henlius benchmarks the highest international standards to construct its quality management system. It has passed nearly one hundred on-site inspections and audits conducted by regulatory authorities and international business partners. Both Xuhui Facility and Songjiang First Plant have been granted with China and the EU GMP certificates. In 2023, the company's manufacturing facilities successively obtained GMP certificates from PIC/S member Indonesia and Brazil for products including HANQUYOU, HANSIZHUANG, and HANLIKANG, and were granted EU GMP certificate for the production lines of HANSIZHUANG. Additionally, the Songjiang First Plant received the Pre-License Inspection (PLI) conducted by the U.S. FDA for the production line of HANQUYOU.
Adhering to patient-centricity, Henlius will further strengthen its commercialization capabilities as a biopharma, deepen its global strategic presence, drive high-quality innovation, and continue to improve the quality and efficiency of its manufacturing operations, so as to achieve a leap from rapid growth to high-quality development, enabling more high-quality innovative outcomes to benefit more patients worldwide.
About Henlius
Henlius (2696.HK) is a global biopharmaceutical company with the vision to offer high-quality, affordable, and innovative biologic medicines for patients worldwide with a focus on oncology, autoimmune diseases, and ophthalmic diseases. Up to date, 5 products have been launched in China, 2 have been approved for marketing in overseas markets, 19 indications are approved worldwide, and 7 marketing applications have been accepted for review in China, the U.S., and the EU, respectively. Since its inception in 2010, Henlius has built an integrated biopharmaceutical platform with core capabilities of high-efficiency and innovation embedded throughout the whole product life cycle including R&D, manufacturing and commercialization. It has established global innovation centers and Shanghai-based manufacturing facilities in line with global Good Manufacturing Practice (GMP), including Xuhui Facility and Songjiang First Plant, both certificated by China and the EU GMP.
Henlius has pro-actively built a diversified and high-quality product pipeline covering over 50 molecules and has continued to explore immuno-oncology combination therapies with proprietary HANSIZHUANG (anti-PD-1 mAb) as backbone. Apart from the launched products HANLIKANG (rituximab), the first China-developed biosimilar, HANQUYOU (trastuzumab for injection, trade name in Europe: Zercepac®), the first China-developed mAb biosimilar approved both in China and Europe, HANDAYUAN (adalimumab) and HANBEITAI (bevacizumab), the innovative product HANSIZHUANG has been approved by the NMPA for the treatment of MSI-H solid tumours, squamous non-small cell lung cancer (sqNSCLC) and extensive-stage small cell lung cancer (ES-SCLC), and esophageal squamous cell carcinoma (ESCC), making it the world's first anti-PD-1 mAb for the first-line treatment of SCLC. What's more, Henlius has conducted over 30 clinical studies for 16 products, expanding its presence in major markets as well as emerging markets.
 产业资讯
产业资讯
 药渡
药渡  2025-11-07
2025-11-07
 66
66
 产业资讯
产业资讯
 药时代
药时代  2025-11-07
2025-11-07
 75
75

 产业资讯
产业资讯
 瞪羚社
瞪羚社  2025-11-07
2025-11-07
 66
66