 研发追踪
研发追踪
 药明康德
药明康德  2019-10-14
2019-10-14
 5803
5803
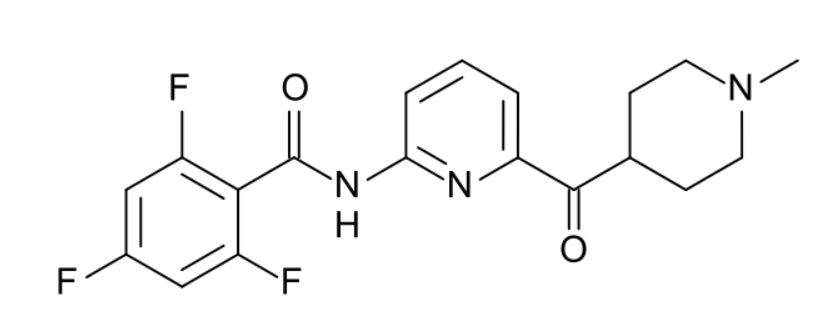
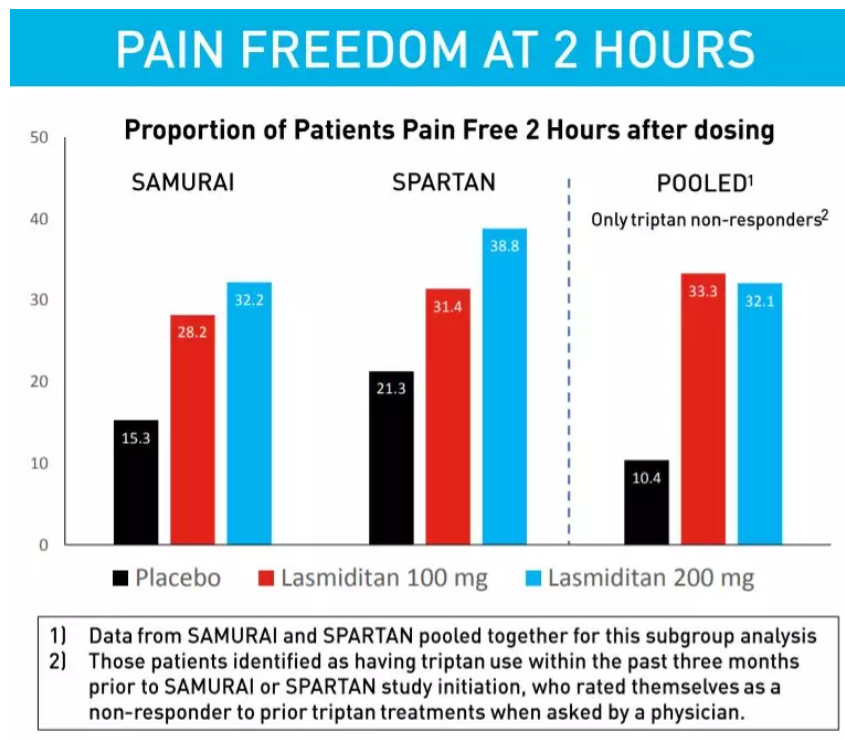
参考资料:
[1] FDA approves new treatment for patients with migraine. Retrieved October 11, 2019, from https://www.prnewswire.com/news-releases/fda-approves-new-treatment-for-patients-with-migraine-300937273.html
[2] Lilly's REYVOW™ (lasmiditan), The First and Only Medicine in a New Class of Acute Treatment for Migraine, Receives FDA Approval. Retrieved October 11, 2019, from https://www.prnewswire.com/news-releases/lillys-reyvow-lasmiditan-the-first-and-only-medicine-in-a-new-class-of-acute-treatment-for-migraine-receives-fda-approval-300937322.html
版权说明:本文来自药明康德内容团队,欢迎个人转发至朋友圈,谢绝媒体或机构未经授权以任何形式转载至其他平台。转载授权请在「药明康德」微信公众号回复“转载”,获取转载须知。

 研发追踪
研发追踪
 瞪羚社
瞪羚社  2025-11-06
2025-11-06
 31
31

 研发追踪
研发追踪
 丁香园Insight数据库
丁香园Insight数据库  2025-11-06
2025-11-06
 30
30

 研发追踪
研发追踪
 药智网
药智网  2025-10-13
2025-10-13
 884
884