 会员动态
会员动态
 复宏汉霖
复宏汉霖  2025-09-10
2025-09-10
 711
711
2025年9月8日,复宏汉霖(2696.HK)宣布,公司自主开发的帕博利珠单抗生物类似药HLX17(重组抗PD-1人源化单克隆抗体注射液)的新药临床试验(IND)申请已经获得美国食品药品监督管理局(FDA)许可,拟用于辅助治疗多种已切除实体肿瘤。
HLX17是复宏汉霖严格按照中国、欧盟和美国等生物类似药法规自主研发的帕博利珠单抗注射液生物类似药,经药学比对,临床前药理学、药效学、药代动力学和免疫原性研究证明,HLX17与原研帕博利珠单抗相似。
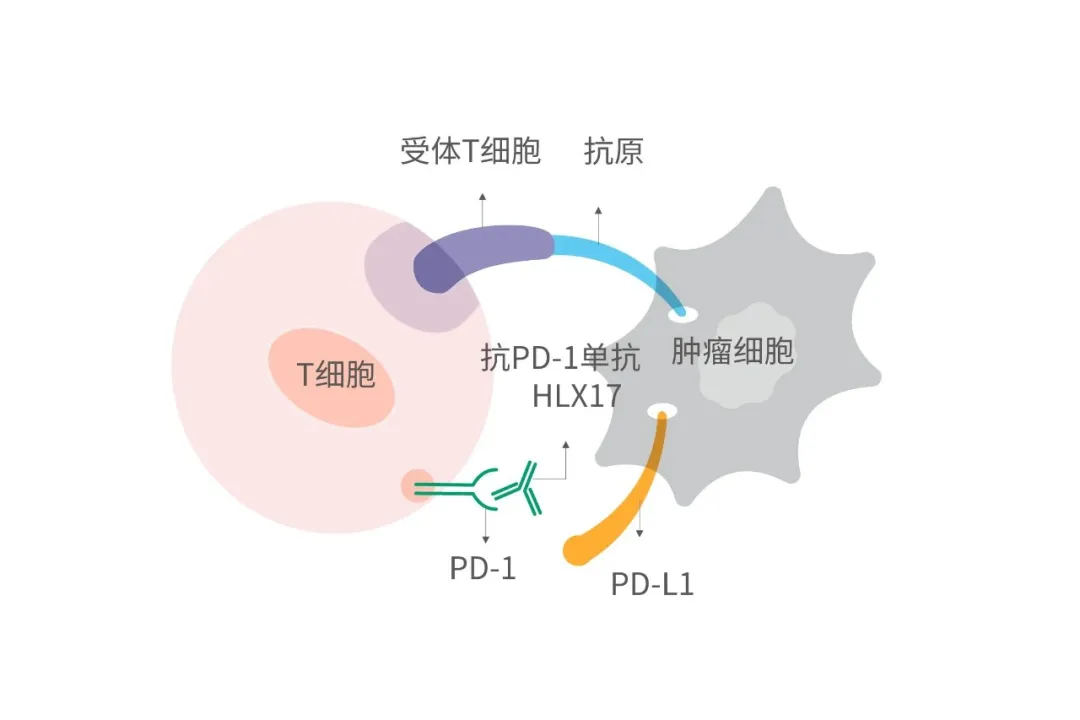
近年来,免疫疗法为肿瘤治疗提供了新的途径,其独特的治疗优势和巨大潜力也陆续得以验证。通过与T细胞上的PD-1受体结合,HLX17能够阻断PD-1与肿瘤细胞上的PD-L1、PD-L2的相互作用,解除PD-1通路介导的免疫应答抑制,包括抗肿瘤免疫应答,从而恢复T细胞对肿瘤的免疫监视和杀伤能力,使肿瘤调亡。在术后辅助治疗中,这一机制有助于清除术后残留的癌细胞,降低复发风险。立足于公司在抗体药物和抗体偶联药物领域的一体化平台优势,复宏汉霖加速免疫治疗和抗体偶联药物的开发和布局,围绕PD-1/L1、CTLA-4、LAG-3等靶点打造出丰富的产品管线,不仅有望在更多适应症中取得突破,也为后续与公司其他产品的协同以及与创新疗法的联合奠定了坚实基础。
未来,复宏汉霖将继续聚焦未满足的临床需求,持续拓宽公司在更多疾病领域的前瞻性布局,为全球患者带去高品质、可负担的创新治疗方案。
关于复宏汉霖
复宏汉霖(2696.HK)是一家国际化的创新生物制药公司,致力于为全球患者提供可负担的高品质生物药,产品覆盖肿瘤、自身免疫疾病、眼科疾病等领域,已在全球获批上市9款产品,5个上市申请分别获中国药监局、美国FDA和欧盟EMA受理。自2010年成立以来,复宏汉霖已建成一体化生物制药平台,高效及创新的自主核心能力贯穿研发、生产及商业运营全产业链。公司已建立完善高效的全球创新中心,按照国际药品生产质量管理规范(GMP)标准进行生产和质量管控,不断夯实一体化综合生产平台,其中,公司商业化生产基地已相继获得中国、欧盟和美国GMP认证。
复宏汉霖前瞻性布局了一个多元化、高质量的产品管线,涵盖约50个分子,并全面推进基于自有抗PD-1单抗H药汉斯状®的肿瘤免疫联合疗法。截至目前,公司已获批上市产品包括全球首个获批一线治疗小细胞肺癌的抗PD-1单抗汉斯状®(斯鲁利单抗,欧洲商品名:Hetronifly®)、自主研发的中美欧三地获批单抗生物类似药汉曲优®(曲妥珠单抗,美国商品名:HERCESSI™,欧洲商品名:Zercepac®)、国内首个生物类似药汉利康®(利妥昔单抗)、以及地舒单抗生物类似药Bildyos®和Bilprevda®。公司亦同步就19个产品在全球范围内开展30多项临床试验,对外授权全面覆盖欧美主流生物药市场和众多新兴市场。
U.S. FDA Clearance of IND for Pembrolizumab Biosimilar HLX17
Shanghai, China, September 8, 2025 - Shanghai Henlius Biotech, Inc. (2696.HK) announced the investigational new drug (IND) application for its HLX17, a proposed pembrolizumab biosimilar independently developed by the company, was approved by the U.S. Food and Drug Administration (FDA) as an adjuvant therapy for certain resected solid tumors,
HLX17 is a pembrolizumab biosimilar independently developed by Henlius in accordance with the NMPA, EMA, FDA and other international biosimilar guidelines. The pharmacologic comparative study, and preclinical pharmacology study, pharmacodynamics, pharmacokinetics and immunogenicity studies have demonstrated that HLX17 is similar to the reference pembrolizumab.
Immune checkpoint inhibitors are playing a crucial part in immunotherapy, which has emerged in recent years as a novel approach to combating tumor cells and their distinct advantages and enormous potential has been continuously validated. HLX17 is a monoclonal antibody that binds to the PD-1 receptor expressed on T cells and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the anti-tumor immune response, thus restoring the T-cell immune surveillance of tumors and increasing its anti-tumour efficacy. In postoperative adjuvant therapy, this mechanism helps to eliminate residual cancer cells after surgery, thereby reducing the risk of cancer recurrence.
Leveraging its integrated platform advantages in antibody drugs and antibody-drug conjugates, Henlius is accelerating the development of immunotherapies and antibody-drug conjugates, building a diversified product pipeline with high potential immune checkpoints including PD-1/L1, CTLA-4, LAG-3, etc., which are expected to show efficacy in multiple indications while laying a foundation for the synergy with in-house products of the company and other innovative therapies.
Looking forward, Henlius will maintain its focus on unmet medical needs and further broaden the company’s layout in more disease areas, commit to bring high quality and affordable treatments for patients worldwide.
About Henlius
Henlius (2696.HK) is a global biopharmaceutical company with the vision to offer high-quality, affordable and innovative biologic medicines for patients worldwide with a focus on oncology, autoimmune diseases and ophthalmic diseases. Up to date, 9 products have been approved for marketing worldwide, and 5 marketing applications have been accepted for review in China, the U.S. and the EU, respectively. Since its inception in 2010, Henlius has built an integrated biopharmaceutical platform with core capabilities of high-efficiency and innovation embedded throughout the whole product life cycle including R&D, manufacturing and commercialization. It has established global innovation centre and Shanghai-based commercial manufacturing facilities certificated by China, the EU and U.S. GMP.
Henlius has pro-actively built a diversified and high-quality product pipeline covering about 50 molecules and has continued to explore immuno-oncology combination therapies with proprietary HANSIZHUANG (anti-PD-1 mAb) as the backbone. To date, the company's launched products include HANSIZHUANG (serplulimab, trade name: Hetronifly® in Europe), the world’s first anti-PD-1 mAb for the first-line treatment of SCLC, HANQUYOU (trastuzumab, trade name: HERCESSI™ in the U.S., Zercepac® in Europe), a China-developed mAb biosimilar approved in China, Europe and U.S., HANLIKANG (rituximab), the first China-developed biosimilar, and denosumab Bildyos® and Bilprevda®. What’s more, Henlius has conducted over 30 clinical studies for 19 products, expanding its presence in major markets as well as emerging markets.

 会员动态
会员动态
 医药观澜
医药观澜  2026-01-29
2026-01-29
 162
162

 会员动态
会员动态
 先声药业
先声药业  2026-01-29
2026-01-29
 160
160

 会员动态
会员动态
 药怪站住
药怪站住  2026-01-28
2026-01-28
 181
181