 研发追踪
研发追踪
 药怪站住
药怪站住  2025-11-17
2025-11-17
 561
561
KURA ONCOLOGY 和 KYOWA KIRIN 宣布 FDA 批准 KOMZIFTI™(ZIFTOMENIB),这是第一个也是唯一一个针对复发或难治性 NPM1 突变急性髓系白血病成人的每日一次靶向治疗。
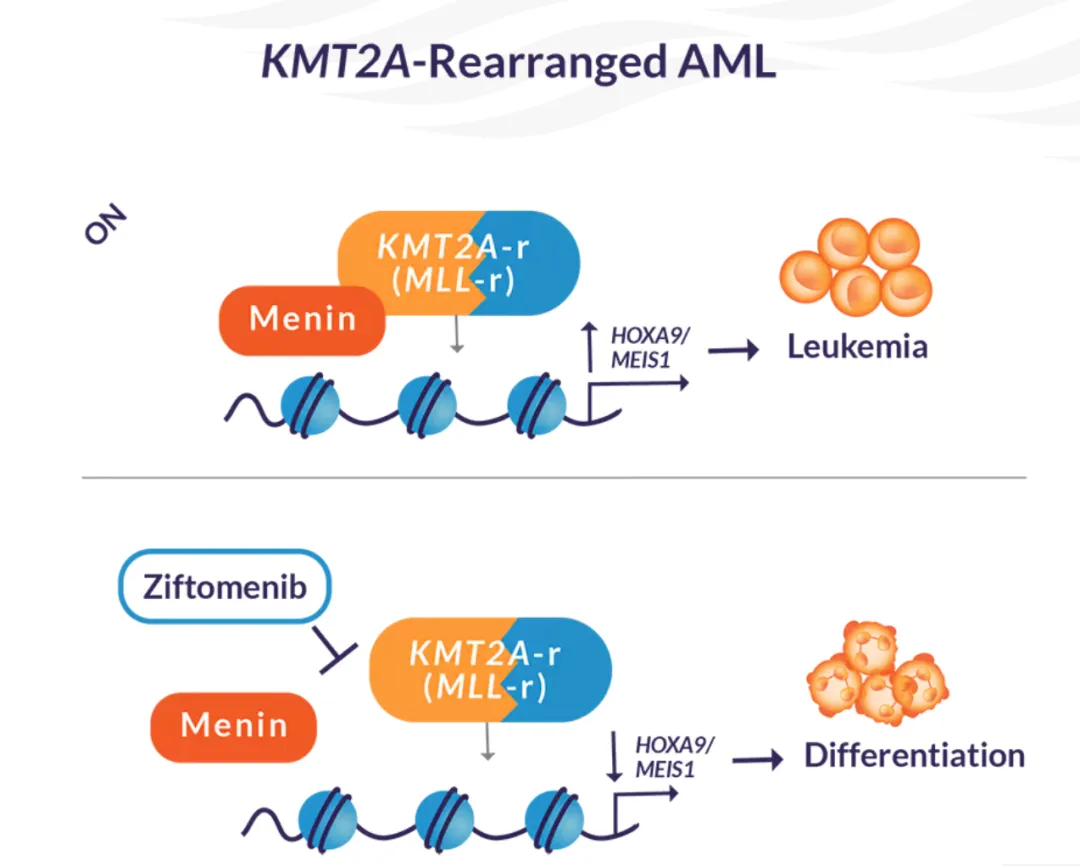
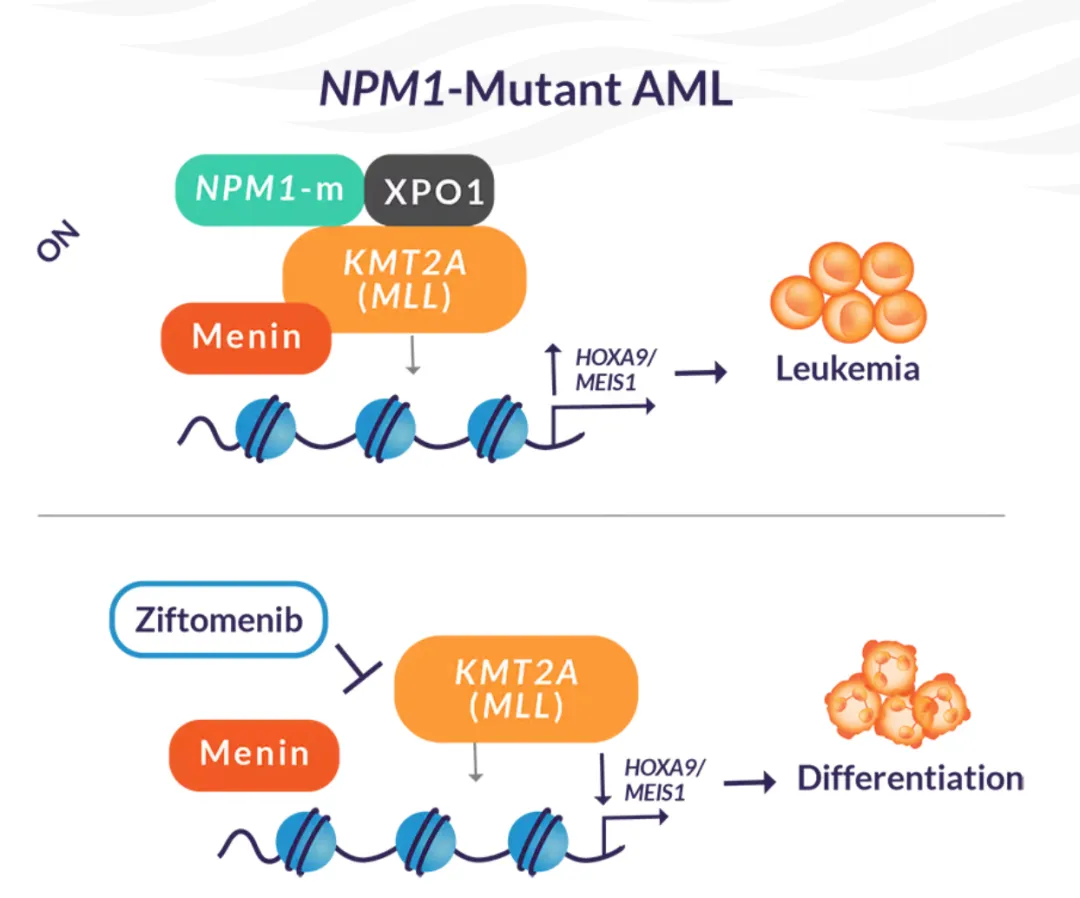
也是第二款Menin抑制剂,第一款为2024年11月美国批准的revumenib(Revuforj、Syndax)。
Menin 是一种调节蛋白,通常有助于血细胞分化;遗传畸变破坏了其正常活动,导致未成熟细胞不受控制地生长。Menin 抑制剂阻断异常活动,有助于恢复正常分化并帮助抑制白血病增殖。
基于 KOMET-001 试验, 92 名患有 NPM1 突变的 R/R AML 成人患者。受试者的中位年龄为 69 岁,之前接受过两线治疗的中位数,其中包括 59% 的维奈托克和 24% 的移植。Ziftomenib 的剂量为 600 mg,每天一次。突变状态由当地临床实验室确定。
KOMZIFTI 在 R/R NPM1 突变 AML 成年患者中表现出深度反应、潜在的同类最佳安全性(没有与 QTc 延长或尖端扭转型室性心动过速相关的黑框警告)、每日一次给药(可与该线常规SOC联用),FDA 在 PDUFA 目标行动日期之前完全批准 KOMZIFTI。
NPM1 突变是 AML 中最常见的创始突变之一,发生在大约 30% 的病例中。从历史上看,大约 20% 的 NPM1-m AML 患者对一线治疗没有反应。在做出反应的人中,70% 的人会在 3 年内复发,大多数会在 12 个月内复发。每次复发的早期复发和生存率下降凸显了对能够实现持久缓解的治疗方法的迫切需求。
该批准得到了关键性 KOMET-001 试验 (NCT04067336) 的支持,该试验评估了 KOMZIFTI 在 112 名 R/R NPM1-m AML 患者中的安全性和有效性。
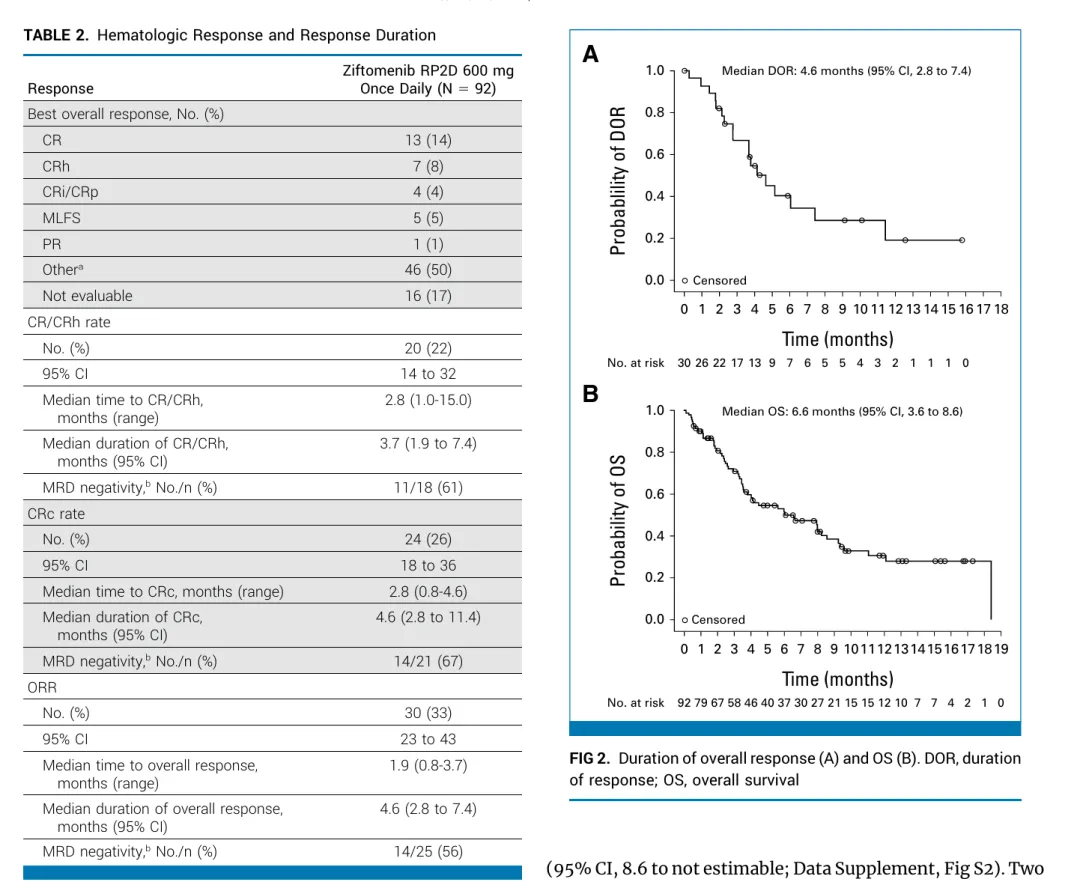
完全缓解率(CR)加 CR 伴部分血液学恢复率(CRh)为 21.4%(95%CI:14.2,30.2),横向高于历史复发NPM1-mAML的12%。CR+CRh 的中位持续时间为 5.0 个月(95% CI:1.9,8.1),达到 CR 或 CRh 的患者首次缓解的中位时间为 2.7 个月(范围:0.9-15 个月)。
响应患者mOS高达18.4m,但是无反应患者mOS仅3.5m。
最常见的不良反应(≥20%),包括实验室异常,是天冬氨酸转氨酶升高、无病原体感染、钾降低、白蛋白降低、丙氨酸转氨酶升高、钠降低、肌酐升高、碱性磷酸酶升高、出血、腹泻、恶心、疲劳、水肿、细菌感染、肌肉骨骼疼痛、胆红素升高、钾升高、分化综合征、瘙痒、发热性中性粒细胞减少、 转氨酶增加。
3级或更严重的治疗中出现的不良事件包括发热性中性粒细胞减少症(26%)、贫血(20%)和血小板减少症(20%)。四分之一的患者出现分化综合征,15% 的患者为3级。两名患者因分化综合征停止治疗,一名患者因呕吐而停止治疗。没有与齐托美尼相关的死亡。
KOMZIFTI 包括分化综合征的黑框警告,这是一种经过充分研究的基于机制的药物中恢复分化的风险。
Kura 计划将其 menin 抑制剂开发用于一线 AML 的两项三期 。1)与维奈托克/阿扎胞苷联合用于具有 NPM1 突变的成人,2)将其添加到具有 NPM1 突变或 KMT2A 易位的成人的标准阿糖胞苷/柔红比星诱导/巩固化疗中。
2025年11月JCO全文发布注册研究结果

Wang ES, Montesinos P, Foran J, Erba H, Rodríguez-Arbolí E, Fedorov K,
Heiblig M, Heidel FH, Altman JK, Baer MR, Ades L, Pettit K, Peterlin P,
Papayannidis C, Berthon C, Walter RB, Shah MV, Balasubramanian S, Khawandanah M,
Salamero Garcia O, Bergeron J, Madanat YF, Roboz GJ, Ulrickson M, Redner RL,
McCloskey J, Pigneux A, de la Fuente Burguera A, Mitra A, Soifer HS, Tabachri M,
Zhang Z, Riches M, Corum D, Leoni M, Issa GC, Fathi AT; KOMET-001. Ziftomenib in
Relapsed or Refractory NPM1-Mutated AML. J Clin Oncol. 2025
Nov;43(31):3381-3390. doi: 10.1200/JCO-25-01694. Epub 2025 Sep 25. PMID:
40997296; PMCID: PMC12573682.
Purpose: Ziftomenib-a potent, highly selective, oral menin inhibitor-was well tolerated and demonstrated encouraging clinical activity as monotherapy for relapsed/refractory NPM1-mutated (NPM1-m) and KMT2A-rearranged AML in the KOMET-001 phase I trial.
Methods: In the registration-enabling phase II part of KOMET-001, patients with relapsed/refractory NPM1-m AML received ziftomenib 600 mg once daily. The primary end point was the rate of complete remission with full hematologic recovery (CR)/CR with partial hematologic recovery (CRh).
Results: From January 26, 2023, to May 13, 2024, 92 patients (median age, 69 years [range, 33-84]) were treated. The primary end point was met, with a CR/CRh rate of 22% (95% CI, 14 to 32; P = .0058); 61% were negative for measurable residual disease. Overall response rate was 33% (95% CI, 23 to 43), with a median duration of 4.6 months (95% CI, 2.8 to 7.4). Prespecified subgroup analyses showed comparable CR/CRh regardless of previous therapy, including venetoclax, or type of comutations. Median overall survival was 6.6 months (95% CI, 3.6 to 8.6). Common grade ≥3 treatment-emergent adverse events were febrile neutropenia (26%), anemia (20%), and thrombocytopenia (20%). Differentiation syndrome occurred in 25% of patients (15% grade 3; no grade 4-5) and was manageable with protocol-defined mitigation. Three patients (3%) discontinued treatment because of ziftomenib-related adverse events.
Conclusion: Ziftomenib demonstrated significant clinical benefit and deep responses in patients with heavily pretreated, relapsed/refractory NPM1-m AML. Ziftomenib was well tolerated with a safety profile consistent with previous studies, including manageable differentiation syndrome, lack of clinically significant QTc prolongation, and low rates of myelosuppression.
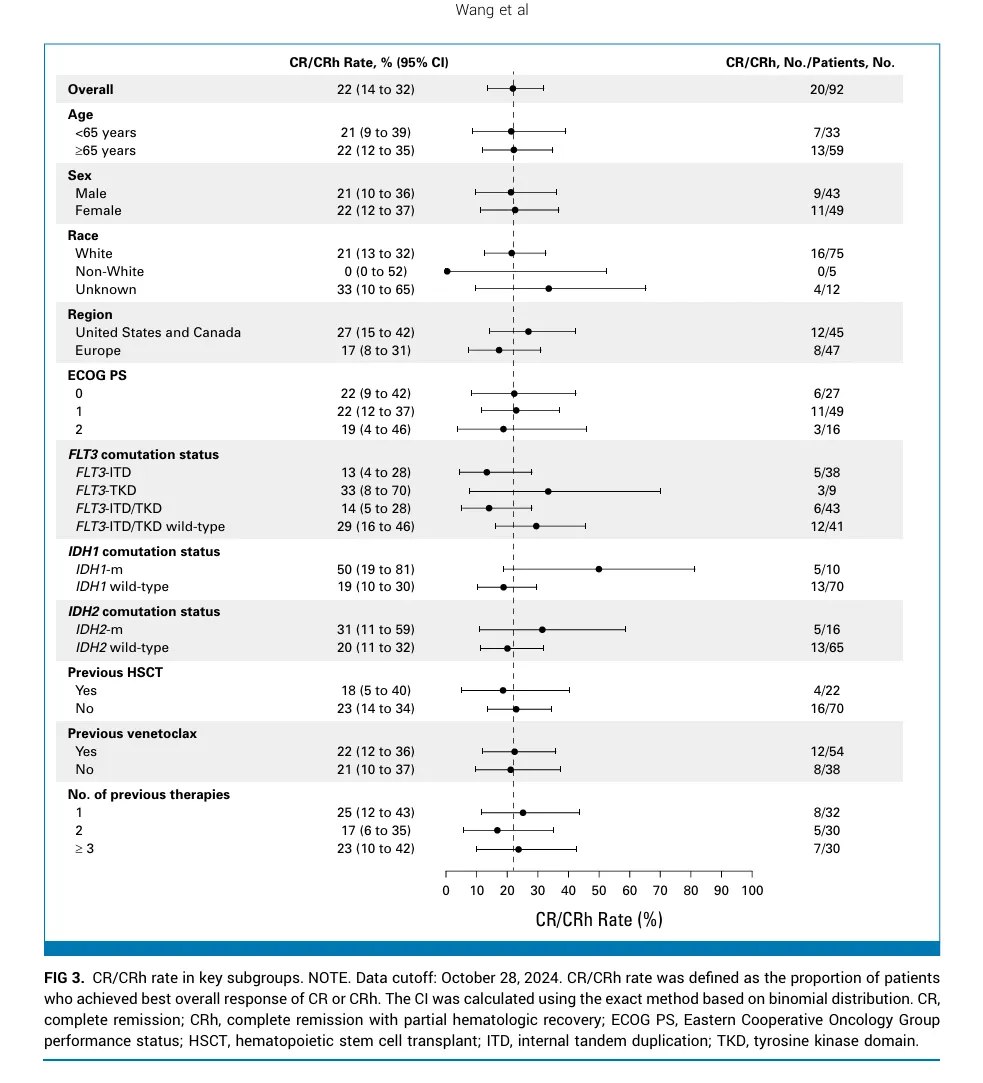
横向与revumenib AUGMENT-101 trial结果比,CR/CRh率相似(22% vs 23%),mOS数值略高(6.6 m vs 4.0
m),但是安全性更优,QT间期延长(13% vs 43%),TRAE血液毒性更低(贫血 5% vs 15%,FN <5% vs
14%,血小板减少<5% vs 10%
公司管线
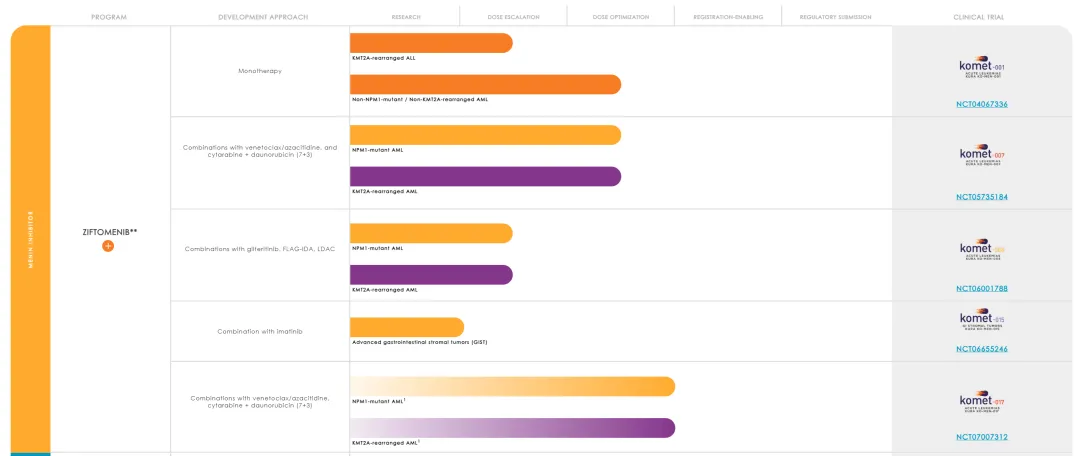

原文链接:

 研发追踪
研发追踪
 医麦客
医麦客  2026-02-12
2026-02-12
 22
22

 研发追踪
研发追踪
 研发客
研发客  2026-02-12
2026-02-12
 19
19

 研发追踪
研发追踪
 丁香园Insight数据库
丁香园Insight数据库  2026-02-12
2026-02-12
 19
19