 产业资讯
产业资讯
 药明康德
药明康德  2025-11-19
2025-11-19
 7
7
编者按:随着精准医学的不断发展,如何将治疗性药物高效且特异性地递送至病灶组织,已成为新药研发的重要课题。通过将能与目标组织中特异蛋白或受体结合的配体与治疗性载荷进行偶联,科研人员已在临床实践中探索出一类兼具安全性与疗效的策略。在常见的单克隆抗体之外,小分子、多肽、寡核苷酸等分子也可作为靶向配体,实现药物的精准递送。然而,这类偶联策略在提升靶向性的同时,也伴随着复杂的化学合成挑战。药明康德在化学业务上的丰富经验为开发这类新一代疗法打下坚实的基础。旗下WuXi TIDES全面的平台能力可为复杂的偶联药物提供一体化解决方案。本文将聚焦偶联药物增强药物组织特异性递送的多种模式,并介绍WuXi TIDES如何通过一体化CRDMO平台,助力合作伙伴加速偶联药物的开发进程。
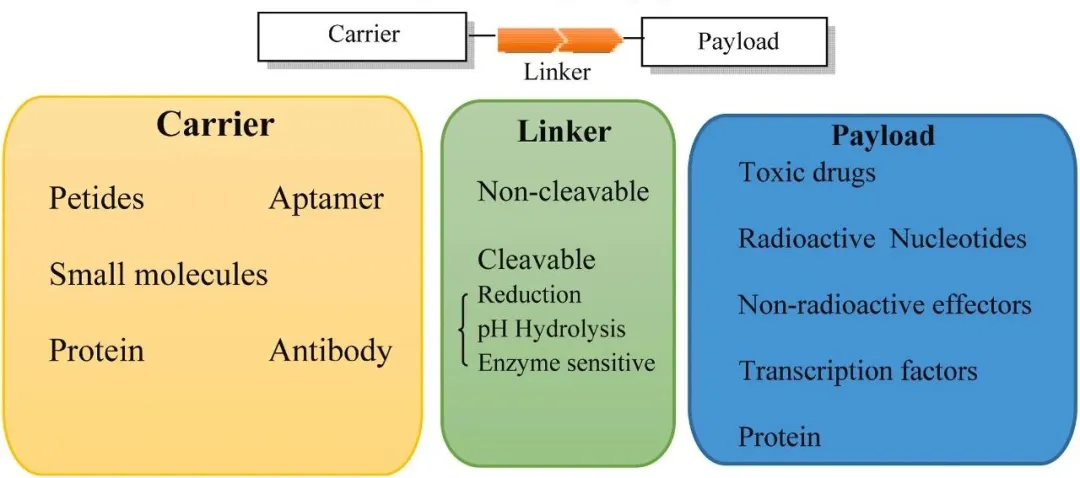
偶联药物的核心结构包括三部分:用于识别靶点的配体、具有治疗活性的有效载荷,以及连接两者的连接子。配体可特异性识别并结合靶细胞表面高表达的受体,进而触发受体介导的内吞作用,将药物高效递送至细胞内部。与传统的被动靶向或全身暴露相比,偶联策略能够在分子层面“导航”药物,在提高疗效的同时显著降低毒性。目前,全球已有近20款抗体偶联药物(ADC)获批上市。值得一提的是,除了传统的ADC,近年来采用小分子、多肽或寡核苷酸作为靶向配体的新一代偶联药物也在快速发展,其所搭载的有效载荷涵盖传统细胞毒性化合物、放射性同位素以及寡核苷酸等创新治疗手段,呈现出多样化趋势。

图片来源:123RF
例如,N-乙酰半乳糖胺(GalNAc)是一种天然糖类结构,能够特异性结合肝细胞表面丰富表达的去唾液酸糖蛋白受体(ASGPR),被广泛应用于肝脏疾病治疗药物的递送。在siRNA和反义寡核苷酸(ASO)药物中,GalNAc偶联策略在递送效率和组织靶向性方面表现尤为突出。已有多款GalNAc偶联寡核苷酸药物获得FDA批准,它们不仅支持皮下注射、患者依从性好,还能高效富集于肝细胞,降低所需剂量,同时具有良好的安全性。
多肽也是一类重要的靶向配体。相比抗体,多肽具有分子量小、组织穿透能力强、易于化学合成等优势,成为新一代偶联药物研发的关键方向之一。通过靶向肿瘤组织中过表达的整合素、生长抑素受体(SSTR)或促性腺激素释放激素受体(LHRH)等靶点,多肽偶联药物可实现精准打击肿瘤细胞。例如,诺华(Novartis)开发的Lutathera(177Lu-dotatate)是一款多肽偶联放射性药物,通过靶向SSTR递送核素,用于治疗神经内分泌肿瘤。在研发管线中,ITM Isotope Technologies的177Lu-edotreotide在胃肠胰神经内分泌肿瘤的3期临床试验中达到主要终点,近日已递交新药申请(NDA)。此外,Bicycle Therapeutics公司致力于双环肽疗法,其多款靶向nectin-4和EphA2等靶点的双环肽偶联药物已进入临床阶段,用于递送细胞毒性或放射性药物,精准杀伤肿瘤细胞。
在寡核苷酸药物递送方面,多肽同样展现出广阔前景。Bicycle公司已与Ionis Pharmaceuticals达成合作,联合开发靶向转铁蛋白受体的双环肽,用于将ASO药物递送至肌肉及中枢神经系统,提升疗效与组织特异性。
▲不同偶联药物类型(图片来源:参考资料[4])
除了小分子和多肽,适配体也是一种有潜力的靶向配体。适配体由单链DNA或RNA构成,具备三维结构,能以高亲和力和特异性与靶点结合,被誉为“化学抗体”。目前已有两款适配体药物获得FDA批准,用于治疗年龄相关性黄斑变性。由于其分子量小、结构可控、免疫原性低等特点,适配体非常适合用于偶联药物的构建。多个适配体偶联抗癌药物正在临床开发中,旨在提升细胞毒性药物的肿瘤特异性递送效率,并进一步探索其在寡核苷酸或纳米结构精准递送方面的应用潜力。
药明康德旗下WuXi TIDES搭建了独特的CRDMO平台,为全球合作伙伴开发寡核苷酸、多肽药物及相关化学偶联物(TIDES药物)提供高效、灵活和高质量解决方案。在多肽偶联药物开发方面,其全面的多肽平台结合了小分子化学能力,支持多肽-毒素、多肽-金属、多肽-GalNAc、多肽-寡核苷酸和放射性核素偶联药物等偶联药物的开发。WuXi TIDES的一体化平台让多个团队能够并行攻关,密切合作,显著提高项目推进速度。下面的这个案例将展示这一平台如何助力合作伙伴,加速一款环肽-GalNAc偶联药物的研发进程。
一体化平台赋能多肽偶联药物开发
在该案例中,合作伙伴的目标是将一款处于发现阶段的环肽-GalNAc偶联药物推进至IND申请。然而,这一过程面临多重挑战:由于药物分子结构复杂,并且合成过程中一个关键多肽中间产物溶解度很低,导致整体产率偏低。同时,合作伙伴以产品的最终商业化为核心目标,这也意味着在早期开发中,如何开发具有成本效益的生产工艺成为首要任务之一。
针对这些挑战,WuXi TIDES的原料药和制剂与分析团队紧密协作,开展联合攻关。首先要解决的是总产率偏低的问题。研发团队利用WuXi TIDES内部的GalNAc合成能力,采取了一系列质量控制措施,成功解决了GalNAc原料中的关键性杂质问题。团队通过添加合适的配体等举措,为提高关键多肽中间产物的溶解度奠定了坚实基础。通过对合成步骤的多重优化,团队将总产率从发现阶段的10%提高至20%,提升幅度高达100%。凭借多个团队的高效协作,WuXi TIDES仅用12个月便顺利将该药物推进至IND申报阶段。客户对产率的显著提升非常满意,并决定继续与WuXi TIDES团队进行GMP生产合作。
在确保顺利推进的同时,WuXi TIDES团队还致力于降低药物生产成本。他们对环肽-GalNAc偶联药物的生产和纯化流程进行了系统性优化。通过替换昂贵的原材料,使原材料成本降低8%;将关键中间产物的生产步骤由8步缩减为7步,使整体生产成本再降低10%;并利用多柱逆流溶剂梯度纯化(MCSGP)技术,在缩短生产周期的同时进一步降低了成本。最终,凭借这一系列持续优化措施,与早期的1公斤GLP批次相比,生产8公斤GMP批次时,每公斤的成本降低了71%。

图片来源:123RF
WuXi TIDES同样可以为复杂的寡核苷酸偶联药物提供一站式解决方案,支持从药物发现到CMC开发再到商业化⽣产。平台能力支持寡核苷酸与GalNAc、多肽、适配体、脂质、药物和其它定制小分子的偶联。
通过兼顾“精准靶向”与“有效治疗”,偶联药物为新药研发带来了全新思路。丰富的配体与载荷选择不仅提升了组织特异性,还拓展了药物类型与适应症的边界。未来,WuXi TIDES将继续依托其一体化、端到端的CRDMO平台,支持合作伙伴推进包括多肽偶联药物、寡核苷酸偶联药物在内的多类偶联药物研发,助力前沿科技转化为惠及全球患者的突破性疗法。
CRDMO: New Conjugates Are Expanding the Boundaries of Precision Medicine
As precision medicine continues to advance, the ability to efficiently and specifically deliver therapeutic agents to diseased tissues has become a critical focus in drug development. One promising strategy involves conjugating therapeutic payloads with ligands that can bind to specific proteins or receptors expressed in target tissues. This approach has demonstrated both safety and efficacy in clinical practice. In addition to monoclonal antibodies, small molecules, peptides, and oligonucleotides have also emerged as targeting ligands, enabling precise drug delivery. However, these conjugation strategies often come with significant synthetic complexity. WuXi AppTec’s deep expertise in chemistry has laid a strong foundation for the development of such next-generation therapies. Through its comprehensive WuXi TIDES platform, WuXi AppTec provides integrated solutions for complex conjugates. This article explores various conjugation strategies that enhance tissue-specific drug delivery and highlights how WuXi TIDES accelerates conjugates development through its integrated CRDMO platform.
A typical conjugated drug consists of three core components: a targeting ligand, a therapeutic payload, and a linker that connects the two. The ligand is designed to selectively bind to receptors that are highly expressed on the surface of target cells, triggering receptor-mediated endocytosis and enabling efficient intracellular drug delivery. Compared to passive targeting or systemic exposure, this strategy allows drugs to be guided at the molecular level, improving efficacy while minimizing toxicity. To date, nearly 20 ADCs have been approved globally. Importantly, a new generation of conjugated drugs is emerging. They utilize small molecules, peptides, or oligonucleotides as targeting ligands, and feature a diverse range of payloads, including traditional cytotoxic agents, radioactive isotopes, and oligonucleotides, signaling a broadening of therapeutic modalities.
Among these innovations, N-acetylgalactosamine (GalNAc) has become a widely used ligand for liver-targeted drug delivery. This naturally occurring sugar binds specifically to the asialoglycoprotein receptor (ASGPR), which is abundantly expressed on hepatocytes. The GalNAc conjugation strategy has shown particularly strong performance in siRNA and antisense oligonucleotide (ASO) therapies, with several GalNAc-conjugated oligonucleotide drugs now approved by the FDA. These agents offer the convenience of subcutaneous administration, excellent patient compliance, efficient hepatic accumulation, lower required doses, and favorable safety profiles.
Peptides also serve as highly effective targeting ligands. Compared to antibodies, peptides offer smaller molecular size, better tissue penetration, and ease of chemical synthesis, making them an important focus for next-generation conjugated drug development. By targeting receptors that are overexpressed in tumors, such as integrins, somatostatin receptors (SSTR), or luteinizing hormone-releasing hormone (LHRH) receptors, peptide-drug conjugates can selectively eliminate cancer cells. For example, Lutathera (177Lu-dotatate), developed by Novartis, is a peptide-radionuclide conjugate that targets SSTR for the treatment of neuroendocrine tumors. In the pipeline, ITM Isotope Technologies’ 177Lu-edotreotide has achieved its primary endpoint in a Phase 3 clinical trial for gastroenteropancreatic neuroendocrine tumors. The company recently filed a New Drug Application (NDA). Additionally, Bicycle Therapeutics is advancing multiple clinical-stage programs involving bicyclic peptide conjugates that target antigens such as nectin-4 and EphA2, delivering cytotoxic or radiotherapeutic payloads to precisely kill tumor cells.
Peptides are also showing promise in the delivery of oligonucleotide-based therapies. Bicycle Therapeutics, in collaboration with Ionis Pharmaceuticals, is developing bicyclic peptides targeting the transferrin receptor to deliver ASO drugs to muscle and central nervous system tissues. This approach aims to enhance both therapeutic efficacy and tissue specificity.
In addition to small molecules and peptides, aptamers represent another class of promising targeting ligands. These single-stranded DNA or RNA molecules form three-dimensional structures that bind to target molecules with high affinity and specificity, earning them the moniker “chemical antibodies.” Two aptamer-based drugs have already been approved by the FDA for the treatment of age-related macular degeneration. With their small molecular size, structural controllability, and low immunogenicity, aptamers are ideally suited for the design of next-generation conjugated therapies. Multiple aptamer-drug conjugates are currently in clinical development to improve the tumor-specific delivery of cytotoxic agents. Researchers are also exploring aptamer-based systems for the targeted delivery of oligonucleotides and nanoscale structures.
WuXi TIDES, a specialized CRDMO platform under WuXi AppTec, provides efficient, flexible, and high-quality solutions for the development of oligonucleotides, peptides, and related chemically conjugated molecules—collectively known as "TIDES" drugs. The platform integrates advanced peptide capabilities with small molecule chemistry, supporting various peptide conjugates, including but not limited to: peptide-toxin, peptide-metal, peptide-GalNAc, peptide-PMO, peptide-oligonucleotide, radionuclide drug conjugate (RDC), etc. The platform’s integrated nature enables cross-functional teams to collaborate in parallel, significantly accelerating project timelines. The following case study illustrates how WuXi TIDES’ integrated platform enables partners to accelerate the development of a peptide-GalNAc conjugate therapy.
Integrated Platform Empowering Peptide Conjugate Development
In this project, the partner’s goal was to advance a peptide-GalNAc conjugate drug candidate, still at the discovery stage, toward an IND submission. The program, however, faced several challenges. The molecular structure of the candidate was highly complex, and a critical peptide intermediate in the synthesis process exhibited very poor solubility, leading to low overall yield. At the same time, because the partner prioritized eventual commercialization, establishing a cost-effective manufacturing process became a central objective in early development.
To address these obstacles, WuXi TIDES’ API, Drug Product and Analytical teams worked in close collaboration. The most urgent issue was the low overall yield. By leveraging WuXi TIDES’ in-house GalNAc synthesis capabilities, the R&D team implemented a series of quality control measures that resolved critical impurity issues in the GalNAc raw material. The team improved the poor solubility of a key peptide intermediate by directly using fractions and adding ligands. Through multiple rounds of process optimization, they not only enhanced the solubility of the key peptide intermediate but also doubled the overall yield, from 10% at the discovery stage to 20%. Thanks to efficient cross-team collaboration, WuXi TIDES advanced the program to the IND submission stage within just 12 months. The client, highly satisfied with the improved yield, chose to continue working with the WuXi TIDES team on GMP manufacturing.
In parallel with improving yield, the WuXi TIDES team also focused on reducing manufacturing costs. They conducted systematic optimization of the production and purification processes for the peptide-GalNAc conjugate. Replacing costly raw materials reduced material costs by 8%. Streamlining the production steps of a key intermediate from eight steps to seven further lowered overall costs by 10%. In addition, adopting multi-column counter-current solvent gradient purification (MCSGP) technology not only shortened the production cycle but also reduced costs further. Ultimately, this series of continuous optimizations enabled the team to reduce the cost per kilogram by 71% for an 8 kg GMP batch compared to the earlier 1 kg GLP batch.
WuXi TIDES also offers one-stop solutions for complex oligonucleotide-drug conjugates, supporting projects from discovery and development through to commercial-scale manufacturing. The platform enables the conjugation of oligonucleotides with GalNAc, peptides, aptamers, lipids, therapeutic agents, and other custom small molecules.
By uniting “precise targeting” with “effective therapy,” conjugated drugs offer a new paradigm in drug innovation. The ability to select from a wide variety of ligands and payloads not only enhances tissue specificity but also expands the scope of drug types and indications. Looking ahead, WuXi TIDES will continue to leverage its fully integrated, end-to-end CRDMO platform to support partners in advancing diverse classes of conjugated drugs—including peptide- and oligonucleotide-based conjugates—ultimately helping to transform scientific breakthroughs into life-changing therapies for patients worldwide.

 产业资讯
产业资讯
 药明康德
药明康德  2025-11-19
2025-11-19
 7
7

 产业资讯
产业资讯
 中国医疗保险
中国医疗保险  2025-11-19
2025-11-19
 6
6

 产业资讯
产业资讯
 药智网
药智网  2025-11-19
2025-11-19
 5
5